Eureka, back to spoilage yeasts. Today, I would like to introduce another spoilage yeast called Metschnikowia pulcherrima (anamorph Candida pulcherrima): A killer yeast with applications in the wine industry, available at Lallemand’s (aka Flavia MP346) and results from one of my countless (beer) split batch experiments. I hope there is something in here for everyone. Put on your science hats, take out your pencils & notepads and start reading. Spoiler alert: you have to interpret the results yourself.
Where do I work?
M. pulcherrima can be isolated from grapes, cherries, Drosophila spp. (fruit fly), flowers and spoiled fruits [Kurtzman et al, 2011]. A yeast commonly found in nature then. If you want to know more about M. pulcherrima and its role in the wine production, go to http://wineserver.ucdavis.edu.
What about beer?
I could not find a source where M. pulcherrima is linked to beer or discussed as potential spoilage yeast thereof. This might not be that surprising since beer is, first of all, commonly not brewed with fruits and second, it’s not really the most alcohol resistant nor metabolically advanced yeast in the universe. Apparently, M. pulcherrima seems to have an alcohol tolerance of about 5% [wineserver.ucdavis.edu, 2015]. Not really high compared to brewer’s yeast with levels of around 12% (or even higher).
What is so special about me?
One very special character of M. pulcherrima makes it a very interesting yeast for the food industry: It’s a killer yeast! The killer activity is affecting blue mold (Penicillium sp.), Botrytis cinerea (gray mold) and couple of bacteria, yeasts [Kurtzman et al, 2011]. This inhibition/killer activity seems to be linked to pulcherriminic acid which is able to form insoluble compounds ultimately depleting the medium of iron ions (which are essential for the growth of other microorganisms) [Oro et al, 2014]. Scientists therefore studied efficacies of M. pulcherrima as a biocontrol agent against the molds mentioned above to eventually prolong the storage times of fruits. If you want to know more about the killer activity and the science behind it, go to PubMed and have a look at the publication from M. Sipiczki. I think this is a very nice example to show people not affiliated with science, that spending money to investigate very odd microorganisms may even result in discoveries that can have an industrial application. Or even have an impact on drug developments against pathological molds. Or put the yeast on the radar of a yeast hunter…
Where can you find me?
 As mentioned in the introduction, Lallemand sells a M. pulcherrima product called Flavia MP346. One of the few advantages to brew in Switzerland is the fact, that everything around me is about wine. And getting wine yeasts is therefore rather easy. I therefore got myself a package of said yeast and tried to investigate the impact on beer.
As mentioned in the introduction, Lallemand sells a M. pulcherrima product called Flavia MP346. One of the few advantages to brew in Switzerland is the fact, that everything around me is about wine. And getting wine yeasts is therefore rather easy. I therefore got myself a package of said yeast and tried to investigate the impact on beer.
According to Lallemand, Flavia MP346 is a strain isolated from Chile with the speciality of α-arabinofuranosidase secretion to increase terpenes and volatile thiols to enhance the aroma of a wine. Overall increasing the aroma complexity (mainly fruit components) in the finished product. The yeast is commonly added to the must first followed by a Saccharomyces pitch 24 h later on.
To test if there is any (detectable) effect on aroma complexity in a malt based beverage like beer, I performed a simple split batch experiment. I started with a straight forward Belgian inspired recipe (3 kg Vienna malt, 3 kg Abbey malt and 0.5 kg CaraMunich 2; 24 IBU with Saazer; OG about 1.066, brewed 12. December 2014), and split the wort in two parts. One part fermented with a Saccharomyces (US-05) only, the second part with a dose of M. pulcherrima for 24 h before pitching the same Saccharomyces strain. I was really curious to try this yeast because an effect seemed very unlikely to me since the yeast can mainly ferment glucose (see below) and is incapable of fermenting maltose. Not to forget the rather low alcohol tolerance. Bottled on 12th of January 2015 (TG_control: 1.023 (5.9 ABV), TG_Metschnikowia: 1.024 (5.9 ABV)).
 First tasting performed in June, 2015 (beer approximately five month in the bottle):
First tasting performed in June, 2015 (beer approximately five month in the bottle):
Control:
Aroma: Lots of dark fruits like figs, prunes. Caramel notes as well as burnt sugar components. Very nice!
Appearance: Red-brown color, slightly cloudy, off-white head (see picture on the left). Lots of carbonation.
Flavor: Very similar to aroma. Caramel and malts. Not much else going on (yeast character or whatever).
Mouthfeel: Light body, average carbonation, malty/sweet-caramel/bitter finish. Very nicely balanced.
Overall Impression: Nice bitter:body balance. Very classical and easy to drink. Most of the character originates from choice of malts (caramalts). Not much flavor picked up from US-05 yeast (as planned).
Metschnikowia partial ferment:
Aroma: Different (compared to control): Besides the malt character (caramel, dark fruits, burnt sugar) notes of raspberries, pepper and wild funk (mostly phenolic acids). Very pleasant aroma profile.
Appearance: Red-brown color, slightly cloudy, off-white head (see picture on the left). Lots of carbonation. Similar to control.
Flavor: Similar to aroma without the fruity components (mainly caramel & malt). Finishes with a bitter overhang (balance toward bitter).
Mouthfeel: Very light body (lighter than control), higher carbonation than control (gusher),
Overall Impression: Typical Belgian dark ale with hints of fruits as well as phenolic funk in the nose. Body on the lower end resulting in an overhang of bitterness (not very well-balanced). Seems to have attenuated more than the control resulting in a low body and over-carbonated beer. In the end, I prefer the control. Although the aroma profile of the Metschnikowia beer is nice, it’s a less drinkable beer (my opinion).
So far for the plain results. Lets put them into perspective. According to Lallemand, the yeast is used in wine to promote the fruit components. And I have the feeling that I was able to pick up such an effect in my experiment as well. The beer dosed with M. pulcherrima had pronounced notes of fruits which were not present in the control. The beer had a higher attenuation level resulting in a lower body and a bitter balanced beer.
Great! I will leave the interpretation of the experiment as well as other applications of this yeast to you. The only thing that I don’t recommend is to ferment a beer with M. pulcherrima only. Simply because of its incapability to ferment the most abundant sugars in wort (just in case someone tries that, goes horribly wrong and wants to sue me for that).
Some biochemical stats about me for yeast ranchers
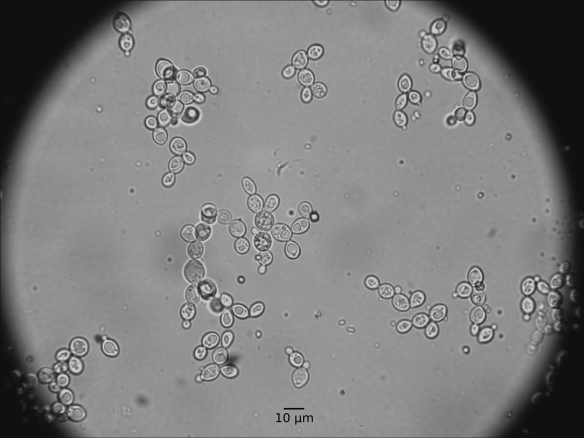
First some micrographs:

 Yeast grows on Sabouraud agar like any other Saccharomyces yeast. I however noticed one difference: M. pulcherrima seems to be non-cycloheximide resistant (according to http://wineserver.ucdavis.edu, 2015). I plated the Lallemand product on Sabouraud agar supplemented with + 10 mg L-1 cycloheximide and I could not detect any colonies. Now some stats summarized from Kurtzman et al (2011).
Yeast grows on Sabouraud agar like any other Saccharomyces yeast. I however noticed one difference: M. pulcherrima seems to be non-cycloheximide resistant (according to http://wineserver.ucdavis.edu, 2015). I plated the Lallemand product on Sabouraud agar supplemented with + 10 mg L-1 cycloheximide and I could not detect any colonies. Now some stats summarized from Kurtzman et al (2011).
| Systematic name: | Metschnikowia pulcherrima (anamorph Candida pulcherrima) | |
| Synonyms: | There are a lots of accepted synonyms for this yeasts. Just some examples: Torula pulcherrima, Saccharomyces pulcherrimus | |
| Growth on malt agar: | Cell morphology: | Globose to ellipsoid, 2.5 µm x 4-10 µm. Pulcherrimin (reddish-brown) pigment present & diffuses into media |
| Clustering: | Not described | |
| Pseudohyphae: | Not described | |
| Pellicle formation: | Not described | |
| Fermentation: | Glucose: | Positive |
| Galactose: | Weak | |
| Sucrose: | Negative | |
| Maltose: | Negative | |
| Lactose: | Negative | |
| Raffinose: | Negative | |
| Trehalose: | Negative | |
That’s all for today. Thanks for reading.
Bibliography
- Kurtzman CP, Fell JW, Boekhout T (2011) The Yeasts, a Taxonomic Study. Volume 1. Fifth edition. Elsevier (Link to sciencedirect)
- Oro L, Ciani M, Comitini F (2014) Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J Appl Microbiol. 116(5):1209-17
- wineserver.ucdavis.edu (2015) http://wineserver.ucdavis.edu/industry/enology/winemicro/wineyeast/metschnikowia_pulcherrima.html