Welcome back everyone. Yes, I am still alive. Although my job in science absorbs lots of my spare time lately, I still find time now and then to brew on a semi-professional scale. Which unfortunately leaves yeast science and this blog at lower priorities. I still do yeast work at home but it all shifted to more practical applications like establishing and testing blends, evaluating yeast isolates and playing around with some full size wine barrels.
What I want to share today are the results of an evaluation experiment I performed a couple of months ago to look for MRS media alternatives to propagate lactobacillus at home. MRS media is kind of the golden standard used to propagate lactobacillus. It works very well but with the disadvantage of being a quite expensive media. I therefore tested a couple of cheaper alternative media and compared the growth/propagation efficiency with MRS. Please notice that the experiment and results are added in a rather short kind of way. That’s all from me now. Take out your pencils & notepads and start reading. Over&Out.
Goal of project
- Set up starter conditions and protocols to propagate Lactobacillus sp. to pitchable amounts
- Compare growth properties to MRS broth as reference
- Determine most efficient media to propagate Lactobacillus sp.
Material & Methods
The following media were tested:
- Lactobacillus media 1: 100% apple juice
- Lactobacillus media 2: 100% apple juice + CaCO3 (20 g L-1)
- Lactobacillus media 3: 100% apple juice + CaCO3 (20 g L-1) + yeast nutrients
- Lactobacillus media 4: 10°P DME
- Lactobacillus media 5: 10°P DME, 10% (v/v) apple juice
- Lactobacillus media 6: 10°P DME, 10% apple juice + CaCO3 (20 g L-1)
- Lactobacillus media 7: 10°P DME, 10% apple juice + CaCO3 (20 g L-1) + yeast nutrients
- MRS-Bouillon (as reference, CarlRoth prepared according to manual)
10°P DME starter and MRS media was autoclaved (15 min at 121°C) and mixed with additional components at room temperature. Pasteurized apple juice was used. Each media (50 mL in total) was inoculated with 1 mL of bacteria culture (Wyeast 5335 L. delbruecki/L. buchneri; Wyeast 5223 L. brevis). Propagation performed at room temperature (no shaking, no aeration).
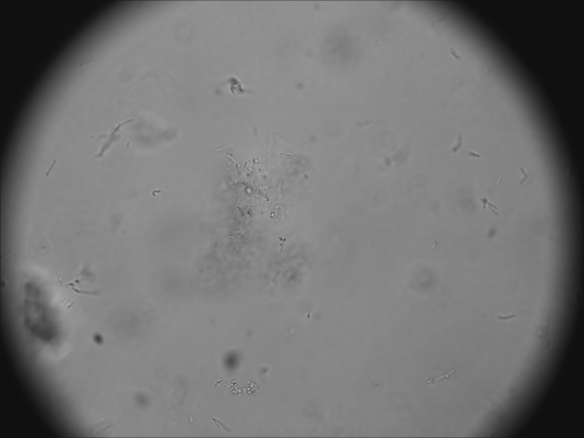
To address the efficiency of the media, the culture densities were estimated based on microscope observations after 7 days of propagation.
Results
WY 5335 L. delbrueckii/L. buchneri:
- Media 1: None-few LAB cells (rod-shaped) visible
- Media 2: Yeast & circular bacteria cells visible (contamination)
- Media 3: Circular bacteria visible (very few LABs)
- Media 4: LAB visible
- Media 5: LAB visible
- Media 6: LAB visible
- Media 7: Lots of LAB
- MRS control: Lots of LAB maybe more than media 7
WY 5223 L. brevis:
- Media 1: None-few LAB
- Media 2: Circular cells, few rod-shaped bacteria
- Media 3: Circular cells, few rod-shaped bacteria
- Media 4: LAB visible
- Media 5: LAB visible, more or less the same as for media 4
- Media 6: Lots of LAB
- Media 7: Lots of LAB, maybe same as media 6
- MRS control: Lots of LAB, same as media 7
pH-measurements after propagation
Unfortunately, I was not able to measure the pH of the media prior to the propagation. Just received my fancy pH-meter a bit to late for that. Below the pH measurements of the media after propagation.
Media // L. delbrueckii // L. brevis
1 // 3.21 // 3.23
2 // 5.80 // 5.87
3 // 6.54 // 5.92
4 // 4.08 // 3.32
5 // 3.10 // 3.22
6 // 5.68 // 4.57
7 // 5.42 // 4.82
MRS // 4.18 // 4.44
Summary & Conclusions
- LAB grow very well in MRS media (room temperature, no aeration)
- Both LAB samples tested grew in various of the tested media. Apple juice, even in presence of other components, does not lead to optimal growth efficiencies compared with the MRS controls
- Propagation in Lactobacillus Media 7 (10°P DME + 10% (v/v) apple juice + 2% (w/v) CaCO3 and yeast nutrients leads to growth efficiencies close to MRS media
In conclusion, growing/propagating LAB in Lactobacillus Media 7 seems to be the most efficient media tested in this series with results similar to MRS media.









Did you take a pH reading of media 7?
Very interesting experiment.
Thank you.
You just reminded me that I did measure the pH values. Added the measurements above. Will visualize the measurements tomorrow to make it a bit easier to read.
Good to hear from you. Darn day job does get in the way sometimes.. 😀
Great post. I’ve also wondered if there wasn’t a cheaper/more efficient growth option than MRS. Since media 7 showed the most consistent growth regardless of LAB, I wonder if controlling for the use of yeast nutrient (paired with both sugar options separately) is worth exploring. Cheers!
Jeff, thanks for reading. Sure one could further play around with the media. I played around myself for a while but stopped. Medium 7 did the trick in the end. Cheers
Interesting that the media with higher growth also had higher final pH. Do you think this is a result of the CaCO3 neutralizing some of the acidity? Do the lactobacillus somehow produce less acidity when conditions are optimal for growth?
Great writeup!
Thanks Derek. The main purpose of the CaCO3 is to neutralize the lactic acid to prevent the pH to drop to low and eventually kill the bacteria. Without CaCO3, the pH can easily drop down to pH 3 (like observed for media 4). And LABs should not produce less if they grow at optimal conditions. The main problem is the high cell densities. More LABs = more acid to lower the pH.
Also, am I correct in my interpretation that you used 20g of CaCO3 in ~50ml of of media? That seems like a lot 🙂
Its 20 g L-1.
Ah, thanks. I’m not familiar with L-1, do you have a reference I can look at?
Sorry. 20 g L-1 is equal to 20 g per Liter of media. Should be 20 g L^(-1) to be absolutely correct. For 50 mL, you would need 1 g of CaCO3 then.
Awesome, thanks so much for putting up with my questions! It’s been quite a few years since I’ve been in a chemistry/biology lab 🙂
Isn’t 20g/L of CaCO3 hard to get into solution?
On my stirplate it looks like milk… when I turn it off it precipitates almost instantly. Am I missing something obvious?
The CaCO3 does not have to be in solution. As soon as acid is present in the media, the CaCO3 solids will slowly go into solution.
Very interesting…
How would you go about applying this to making petri plates? Is there an alternative to actual MRS (which I am finding quite hard to source).
I would start with the base recipe (without CaCO3) and add some agar agar. And give it a spin.
I figure that I could use HLP to make plates to help for selection… but after that how can I get rid of the cycloheximide?
Why not isolate the bacteria from the plates and simply propagate it as usual? Obviously not in HLP media. But I think there is no need for selection in the subsequent propagation anyway. Just go with a MRS media or the options mentioned in the post.
Well lacto doesn’t seem to want to grow on my “normal” plates (10P DME + yeast nutrients + agar). I get a positive HLP test (anaerobic) but when I plate I never seem to get any lacto colonies. I’m still looking for MRS but I was trying to figure out a way to use that media 7 as plating material.
I have good results with MRS plates but no luck with any other homemade agar media so far. And due to my limited free time, I just stick to MRS agar plates and never invested time to find an alternative there. And use my own media to propagate the LABs to pitchable amounts. Maybe try to grow the plates anaerobically?
I could try to propagate with the liquid media … but I’m trying to isolate lacto from a mixed culture and I figure the brett would also grow. Hence the need for an isolating media that lacto actually grows on (and short of actual MRS)
Since you seem to have access to HLP, why not plate the mixed culture on there and re-streak it for a couple of times? Like 4-5 times? This should be enough to enrich for LABs and get rid of the Bretts. Chances you pick up Brett together with a LAB colony gets lower and lower with each re-streak.
I am not equiped for anaerobic incubation sadly..
Where do you get your MRS from? I only found obscure companies I had to send enquiries with… and that never responded. I found HLP, LMDA, WLD/WLN and all sorts of things but MRS eludes me.
Oh and thank you so much for the pointers ;o)
I will publish a post about anaerobic incubation alternatives pretty soon (hopefully by the end of the month).
I got my MRS from a local chemical supplier. Have you tried Amazon?
We get our MRS media (agar and broth) from Fisher Scientific.
I believe that would work for the isolation part indeed. I have never tried to use something off of HLP (fear of the cycloheximide) and I heard that I had to streak a few times afterwards to get rid of it before actual culturing… So I get to the same problem: how do I plate that now isolated lacto without MRS? Or is there other ways of getting rid of the cyclo?
Couple of propagations in liquid media (like the one mentioned in the post above, no cycloheximide) should do the trick. 5 mL cultures?
> how do I plate that now isolated lacto without MRS?
No idea since I never tested anything beside MRS that worked properly.
Here is a cheap anaerobic method for lactobacilli we use all the time my micro class.
Find a jar big enough to put in the petrii dishes (I have been using 60 mm × 15 mm dishes to save on MRS media) so I can use a 1/2 or 1 gallon mason jars. invert the dishes after streaking and then place a candle on top of the dishes and light it. Then seal the jar with airtight mason jar lid You could always wrap the lid lip in plastic wrap to help seal. Incubate 35-37 C couple days. This works for test tube cultures as well. The flame uses up O2 and creates CO2 which helps some lactobacilli grow.
Also wondering if 5.2 Mash Stabilizer (at pH 5.2) could be added to Media 7 instead of CACO3 for making agar plates. It should be soluble. MRS is buffered at pH 6.5 so this would be lower.
🙂 Candle jars is the content of the post I mentioned there …
When you look at the cost for the bulk ingredients and the time to make it, are you seeing much economy over MRS?
Absolutely. I propagate Lactobacillus up to higher pitching rates (50 gal batches) and there is no way I could afford that with MRS.
Thanks, Sam! One question regarding the chalk. I assume that the entire starter should be pitched into the beer/wort that is to be soured, rather than cold crashed, decanted, and then pitched (I’ve read from Bryan Heit that cold crashing can negatively effect the performance of the Lacto). Will that much chalk have an effect on buffering the acid production in the full batch of beer?
Cheers, I commonly leave the starter at room temperature and just decant off the supernatant. Leaving the chalk sediment in the flask. I therefore have no idea about any impact of chalk on acid production on a full batch of beer.
Thanks, Sam! If I understand you correctly, the chalk will eventually sediment out to the bottom of the flask. About how long does that take? Cheers!
You are correct. Since I don’t shake the flask with a stirrer (only by hand once in a while), the chalk sediments rather quickly. Couple of hours maybe?
Thank you, Sam. FYI, I’ve used some of your information here (I linked this article inline and as a reference): http://www.milkthefunk.com/wiki/Lactobacillus#Starters_and_Pitching_Rate. Let me know if there are any issues with this please. 🙂
Sam:
wondering if there is interest in following acid production and lactic acid concentration in actual wort (as you might find in kettle souring) with different species of lacto including some non-traditional ones. Have some students doing total acidity, pH and Liquid chromatography assay for lactic acid concentrations. doing pure cultures in MRS and simple malt extract now.
Roy, this sound very interesting and I think I might not be the only one interested in such results.
nice work by the way.
p.s I found this article (well one page is accessible) which describes the use of CaCO3 to neutralize acid in bacteria media published in 1911 ! http://jama.jamanetwork.com/article.aspx?articleid=448814
Will have a look at it. Sounds interesting, thanks for sharing.
Sam. When you autoclave the DME is it cloudy? I make it in lab nano pure water and it has some precipitate after heating. Interestingly, the malt extract from Difco that I use in micro lab is clear after autoclave get. Guess micro media ME is treated/made differently.
My media is cloudy which is very likely from protein precipitates. Could be that commercial media do not contain (or less) denaturing proteins?!?
Fun stuff. I am doing some bacteria isolation’s from some mixed cultures I have come across over the last couple of years. We have done a couple of LAB Cultures for use in Berliner weisse however I never got the cell density I was expecting in the simple media I was using (mostly DME). Thanks to this post I am going to make some MRS plates and broths and do a couple of experiments to see what concentration of LAB I need to achieve to successfully achieve the acidity I want in a reasonable amount of time. Since pitching rates do not really exist for LAB I will likely try to use CFU to correlate to cell density.
Really interesting blog! Sorry for the “newbie” question… it’s possible to replace CaCO3 with the common sodium bicarbonate obtaining the same lactic acid buffering capacity?
I actually have no idea if that would work. I would at least be cautious to add sodium bicarbonate to the media prior to autoclaving (might generate lots of CO2 due to thermal treatment). If I look at the chemistry correctly, in case of CaCO3, the created calcium lactate has a lower solubility than sodium lactate (in case one would use sodium bicarbonate). And I therefore do not know how good the buffer capacity of sodium lactate is compared to calcium carbonate. Meaning, I cannot tell if the sodium carbonate would be good enough to “capture” the producing lactic acid and keeping the pH at high levels.
Hi, for a science project, I have to grow Lactobacillus but cannot buy MRS agar. For medium 7:(Lactobacillus media 7: 10°P DME, 10% apple juice + CaCO3 (20 g L-1) + yeast nutrients), can you say what exactly is the recipe? How much of each component must be present in the mixture? Thank you in advance,
Hi there,
for one Liter of media you would need: 100 g of dry malt extract (DME), 900 mL of water, 100 mL of apple juice and 20 g of CaCO3 and a tablespoon of yeast nutrients. Start with 100 g of DME and add the 900 mL of water + yeast nutrients. Mix until DME is dissolved and autoclave. When media reaches room temperature, add the 100 mL of apple juice and the 20 g of CaCO3. Just be aware that the CaCO3 will not be dissolved hence don’t worry about the CaCO3 sediment at the bottom. If aliquots of the media are used, mix entire media prior to aliquoting to ensure each aliquot has some CaCO3. Media is then ready for cultivating some Lactos.
What volume of this type of starter would you make for 20 liters of wort? What is the approximate cell density that you hit with this starter method? Thanks again, Sam!
> What volume of this type of starter would you make for 20 liters of wort?
Depends on what you are looking for in terms of acidity, lacto-strain and how you prepare the wort. I commonly did no-boils and worked with 500 mL ad 5 gal batch. Works fine for me and gives me the character I am looking for. However, this heavily depends on the kind of lacto strain one uses (I use a blend of commercial and commercial ones), and feel okay with the pH and profiles I get in the end.
> cell density that you hit with this starter method?
No idea. Never performed a cell count…
A question about the CaCO3 that applies only for making a starter (step-up to pitch in, say, 5 gallons): the amount of chalk seems excessive. I understand that you want some precipitated chalk that can interact with the lactic acid as it is produced. This makes total sense. (Aside: I thought the buffering was provided not by the formation of calcium lactate, which leaves the protons in solution, but by the capture of protons by carbonate (ok, it’s true that the carbonates are liberated during the formation of calcium lactate) to form bicarbonate ions in solution. Maybe I misunderstood some of the above comments, though.) But when I made up a half liter of this solution, I have to say after two days most of the chalk was still precipitated, which means the all that chalk wasn’t needed to grab those pesky protons. Have you experimented with, say, 1g per liter or 5 g per liter to see if you still have plenty of chalk to do all the buffering you need? I only ask because I found decanting off so much chalk to be a waste of starter solution. Otherwise I was very happy with the results of using your solution as a starter for LAB.
> Capture of protons by carbonate
I see it the same way. If I have my chemistry together, it should look something like: CaCO3{s} + 2 CH3CH(OH)COOH{aq} > Ca-lactate{aq} + H2CO3 > H2O{l} + CO2{g}. Whereas the H2CO3 would further react to H2O + CO2.
20 g CaCO3 (= 0.2 mol) can neutralize 0.4 mol of lactic acid (= 36 g, approx. 3.6%), As far as I know, a good souring by LABs can produce up to 1-2% of lactic acid (= 10-20 g of lactic acid in total). With the 20g of CaCO3, I am still in a green zone of buffering.
> Have you experimented with, say, 1g per liter or 5 g per liter to see if you still have plenty of chalk to do all the buffering you need?
I never tried anything else than 20 g. Just keep in mind that 5 g would only capture a 1/4 of the lactic acid (36/4 = 9 g per liter, 0.9%).
Is there any particular reason you didn’t autoclave all ingredients in one shot?
Yes, to keep the vitamins alive in the apple juice.
Pingback: Lactobacillus 2.0 - Advanced Techniques for Fast Souring Beer - Sour Beer Blog
Thanks for your work and sharing, this is great info! I do have a question though. I noticed there were no tests of DME, CaCO3 and yeast nutrients so I was curious what you think the apple juice is contributing to the growth? Do you think the apple juice is a critical component of the mixture? Thanks again!
Hiya,
I think apple juice brings some simpler sugars (compared to DME) as well as vitamins to the table. As for the criticality, I don’t have access to my experimental data but I think I observed a difference in starters with & without apple juice. Apple juice is very likely not super important but might get your Lactos a bit farther than without.
hi,
im wondering why you propgate at room temp and not at 100F?
Hi, sorry for the late reply. Do not check out my blog on a regular basis any more.
> Why you propgate at room temp and not at 100F?
Very simple. I don’t have a incubator.
hi sir this is bhargavi this is very interesting using apple juice and i have one doubt that apple juice is adding after autoclaving then there will be contamination of media because of microbes present in the juice
Hi there bhargavi, what you say is true. However, I work with pasteurized apple juice and furthermore, I am not concerned with contaminations as these will not have a big impact on the final beer itself.
Pingback: Warzenie piw kwaśnych. Część 1: Lactobacillus. — BeerFreak.pl
Why did you use pasteurized apple juice instead of unpasteurized?
If you would go for unpasteurized, you may propagate any bacteria from the apple juice. Which is not what you would like to do here.
What do you think would happen if you go with everything on Media 7 but the apple juice?
Should work as well. I would not be surprised if it would work as well as with an addition of apple juice.